For Biotechs and Pharma professionals
OncoDNA solutions solve problems faced by Pharma with a high level of customization depending on your needs
We understand, Biotechs, Biopharma, and CRO companies are grappling with managing an ever-increasing amount of complex data. At OncoDNA, our mission is to provide unwavering support throughout the entire journey, from drug development to marketed drug, offering an extensive portfolio of products and services tailored to meet your needs.
Pharma professionals can rely on our extensive expertise to access and interpret genomic data for clinical studies
Phase I-III Clinical Trials
Assisting biopharma companies from the early phase I to phase III by testing and assessing real-world patients
We assist biopharma companies in the following:
Phase I
- Early detection of biomarkers
- Patient enrollment
- Patient stratification
- Safety assessment
Phase II
- Confirmation of biomarker validity
- Treatment response monitoring
- Refinement of companion diagnostics
Phase III
- Real-Time Monitoring of Biomarkers
Licensed drugs
Conducting Post-marketing studies to evaluate the long-term effects of the drug after the medicine has received regulatory approval (market authorization)
We assist biopharma companies in the following:
- Long-term safety monitoring
- Monitoring of resistance and relapse
- Real-world effectiveness studies
Transitional studies
Phase I-III Clinical Trials
Licensed drugs
Study Design
Our experts help your company in answering important questions such as sample timing, sample to be collected...
By leveraging Oncodna Solutions' expertise, pharmaceutical companies can design studies with enhanced precision and efficacy. We can stratify patient cohorts based on molecular profiles, enabling more targeted enrollment and facilitate the identification of predictive biomarkers for treatment response.
Logistics
Full support for sample collection & specimen shipment with global capabilities
Logistics in clinical trials encompass a range of activities that are essential for the successful planning, execution, and completion of a study. Efficient logistics contribute to the reliability of study results, participant safety, and overall trial success.
Communication
Transparent engagement plans focused on collaborative problem solving
Due to the large amount of data, OncoDNA provides biopharma companies with insight via our visualization platform where data is integrated, curated, validated and thus reliable.
Quality Control
Focused on managing relevant quality standards for your project
Our approach encourages a collective effort in overcoming obstacles, enhances the quality of research outcomes, and ultimately contributes to advancing medical knowledge and improving patient outcomes.
Analysis & Report
Easy to use reporting tools to provide you with the data you need to evidence your project
We closely monitor and validate the quality of our data, OncoDNA ensures potential issues are promptly identified and resolved, maintaining the credibility of the study.
CLIA-CAP laboratory that meets regulatory requirements for your trials
Based in France our laboratory is CLIA-certified and CAP-accredited, evidencing our commitment to delivering diagnostic services of the highest caliber. Achieving Clinical Laboratory Improvement Amendments (CLIA) certification and College of American Pathologists (CAP) accreditation places us at the forefront of precision and reliability in clinical testing.

Insight into your oncology data
From individual patient...
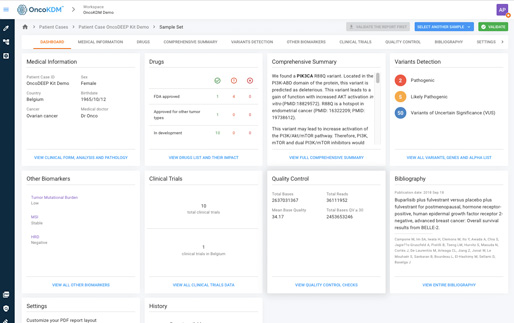
...to global visualization
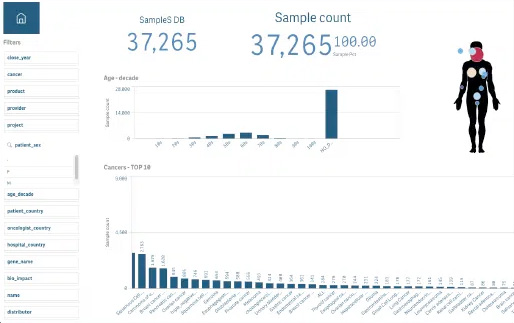
Over the past decade, there has been a rapid acceleration in the innovation of cancer diagnostics, treatments, and patient management. As a result of this acceleration is the generation of a large amount of data. When data is linked, it has the potential to provide oncology experts such as laboratories and pharma companies with comprehensive insights, enabling data-driven decision-making.






