In the ever-evolving landscape of oncology clinical trials, the pursuit of precision has become synonymous with progress. In this era where tailored therapies hold the promise of improved patient outcomes, the foundation of reliable and accurate diagnostics and follow-up becomes paramount. This article explores two critical facets shaping the landscape of clinical trials: the gold standards of CLIA/CAP certification and the transformative trend from solid to liquid biopsy.
 CLIA Certification and CAP Accreditation: highest standards for precision testing
CLIA Certification and CAP Accreditation: highest standards for precision testing
Today, clinical Laboratory Improvement Amendments (CLIA) certification and College of American Pathologists (CAP) accreditation stand as key requirements of precision in oncology clinical studies. The former ensures the highest standards of clinical laboratory testing, guaranteeing accuracy in molecular diagnostics. CAP accreditation, on the other hand, signifies a commitment to unparalleled quality, providing researchers with reliable and consistent results crucial for advancing our understanding of cancer.
Liquid Biopsy: a paradigm shift in clinical trials
Traditional tissue biopsies have limitations (invasive, tumor heterogeneity, tissueavailability, sampling bias, …) and the spotlight is now on liquid biopsy. The non-invasive approach analyzing circulating tumor DNA (ctDNA) and other biomarkers in the bloodstream offers real-time insights into tumor dynamics, treatment response, and the detection of minimal residual disease (MRD), enhancing the clinical trial’s approach. Reasons why OncoDNA has developed liquid biopsy solutions for precision oncology research for more several years.
OncoSELECT®: tailored biomarker profiling
By detecting potential sensitivity or resistance to targeted therapies and hormone treatments OncoSELECT®, empowers researchers with biomarker profiling, aiding in the identification of genetic alterations crucial for patient stratification and the development of personalized treatment plans in oncology clinical studies.
OncoFOLLOW®: real-time treatment monitoring
As demonstrated in two recent studies from Curie Institute (1), OncoFOLLO® allows detection of pathogenic variants that were missing from paired tissue-based NGS results, suggesting spatial heterogeneity but also facilitates real-time monitoring of treatment response. By tracking changes in ctDNA levels, it identifies emerging resistance mechanisms, allowing for adaptive trial design and optimized patient outcomes.
MRD for Pharma: ultra-sensitive detection of residual disease
As depicted below Minimal Residual Disease (MRD) allows for early detection of recurrence and precise measurement of tumor burden (2) (3) (4) (5). This precision enhances the evaluation of treatment efficacy, guiding informed decision-making in clinical trials and ultimately improving patient outcomes in real-time.

Figure 1. Early detection of cancer recurrence comparing to CT approach.
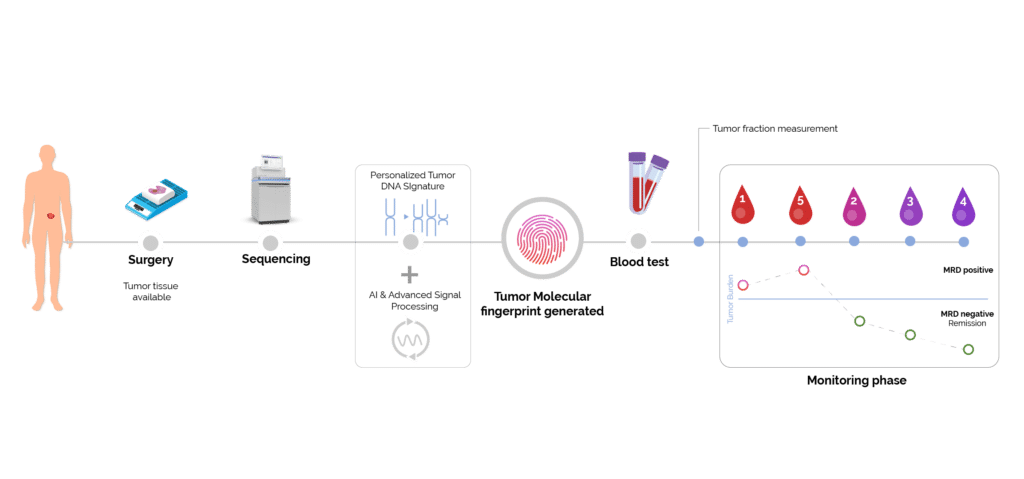
Figure 2. Tumor fraction monitoring.
(5) AstraZeneca extends C2i pact to validate minimal residual disease solid tumor blood test
In conclusion, OncoDNA, with its recently acquired CLIA certification and CAP Accreditation, stands as a great ally for biotech and pharmaceutical companies. It proves to be an invaluable partner, providing comprehensive support throughout the crucial stages of clinical studies, from transitional research to post-market surveillance.
