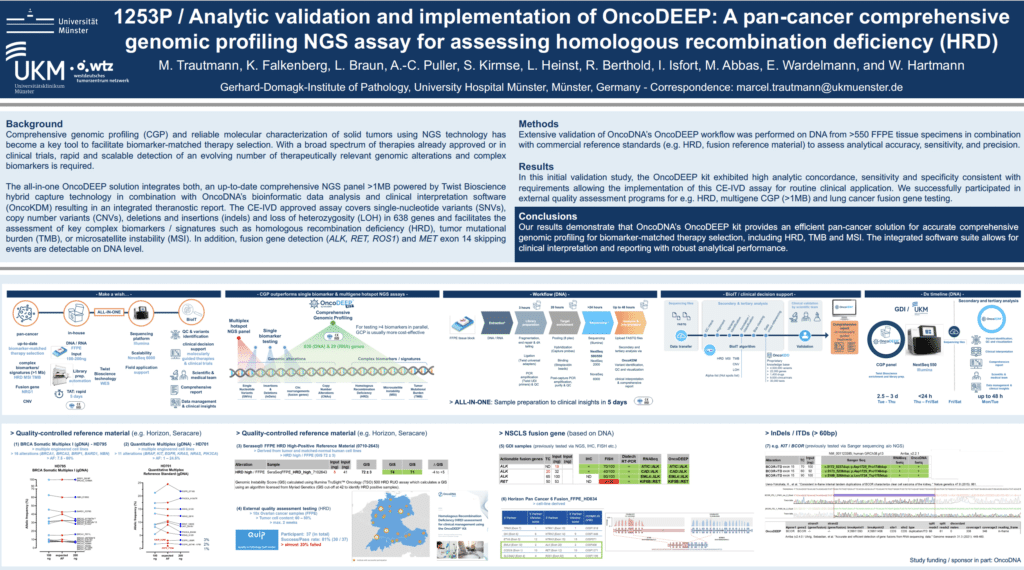
Comprehensive genomic profiling (CGP) and reliable molecular characterization of solid tumors using NGS technology has become a key tool to facilitate biomarker-matched therapy selection. With a broad spectrum of therapies already approved or in clinical trials, rapid and scalable detection of an evolving number of therapeutically relevant genomic alterations and complex biomarkers is required.
The all-in-one OncoDEEP solution integrates both, an up-to-date comprehensive NGS panel >1MB powered by Twist Bioscience hybrid capture technology in combination with OncoDNA’s bioinformatic data analysis and clinical interpretation software (OncoKDM) resulting in an integrated theranostic report. The CE-IVD approved assay covers single-nucleotide variants (SNVs), copy number variants (CNVs), deletions and insertions (indels) and loss of heterozygosity (LOH) in 638 genes and facilitates the assessment of key complex biomarkers / signatures such as homologous recombination deficiency (HRD), tumor mutational burden (TMB), or microsatellite instability (MSI). In addition, fusion gene detection (ALK, RET, ROS1) and MET exon 14 skipping events are detectable on DNA level.