The two key questions addressed by biomarker testing are (1) is there an approved therapy available, and (2) is there a clinical trial option.
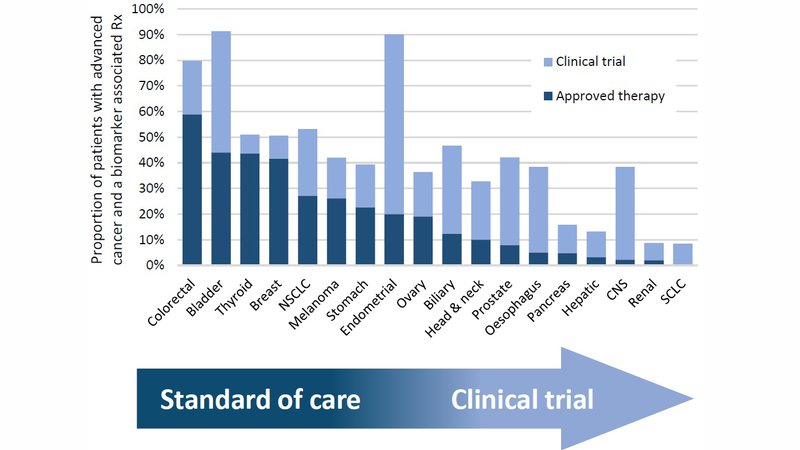
Currently, a quarter of patients with advanced cancer will potentially benefit from a biomarker associated therapy. Similarly, for around a quarter of patients a biomarker stratified clinical trial may be an option It is important to note, however, that this benefit is not distributed equally across all cancers, as shown in the bar chart.
The optimal time for biomarker testing is at the time of diagnosis with advanced disease.
Biomarkers of response to molecularly targeted therapy are almost invariably present in all the cancer cells1 2 and are stable over time. Biomarker testing at diagnosis allows the patient and their physician to map out the treatment pathway, through standard of care to experimental therapies. Where standard of care therapy is suboptimal, patients may choose to move quickly to a clinical trial. Unfortunately, patients and physicians sometimes wait until it is too late before thinking about a clinical trial, by which time the patient may be too unwell. Planning ahead provides a clearer view of the clinical trials available and may allow travel to another hospital in order to access the best trial option. Clinical trials often enroll patients at a specific point in the treatment pathway, for example following a specific line of therapy. Planning ahead ensures that patients are not denied access to a potentially beneficial clinical trial because of a treatment they have already received.
Biomarker associated approved and experimental therapies are not available for all patients. It is important that patients and their physicians understand this before biomarker testing is arranged so their expectations are realistic. Overall, comprehensive biomarker testing will identity either an approved therapy or a clinical trial for around a half of patients with advanced cancer.
1 Nat Rev Cancer. 2019 Nov;19(11):639 650.
2 Nature. 2019 Nov;575(7781):210 216.