Introduction : HRD validation with OncoDEEP® Kit for ovarian cancer
This poster highlights the results of a rigorous study conducted on 76 ovarian carcinoma samples from four Spanish hospitals. The OncoDEEP® kit stands out for its ability to analyze 638 genes and provide critical genomic signatures such as Tumor Mutational Burden (TMB), Microsatellite Instability (MSI), and HRD. The validation results demonstrate an overall concordance of 88.8% with a sensitivity of 90% and specificity of 88% compared to gold standard references, confirming the effectiveness of the OncoDEEP® kit in identifying ovarian cancer patients eligible for PARP inhibitor therapy. By combining the analysis of BRCA1/2 status and genomic markers such as Loss of Heterozygosity (LOH), Allelic Disparity on Telomeres (ADT), and Large-scale Rearrangements (LR), the kit offers a comprehensive solution for tumor characterization, reducing both costs and processing time. Explore this poster to understand how the OncoDEEP® kit is revolutionizing the detection of genomic signatures and improving patient management through advanced technology and dedicated bioinformatics analyses.Figure 1.
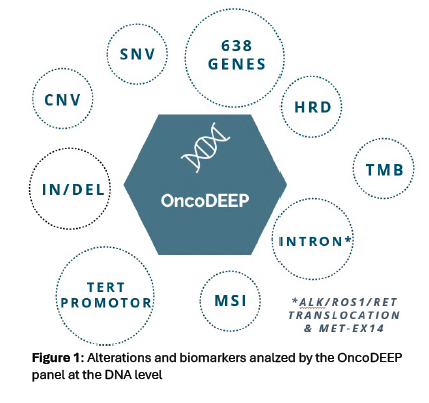

Figure 2.
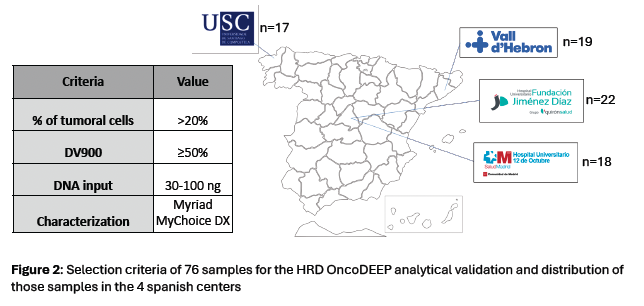

Figure 3.
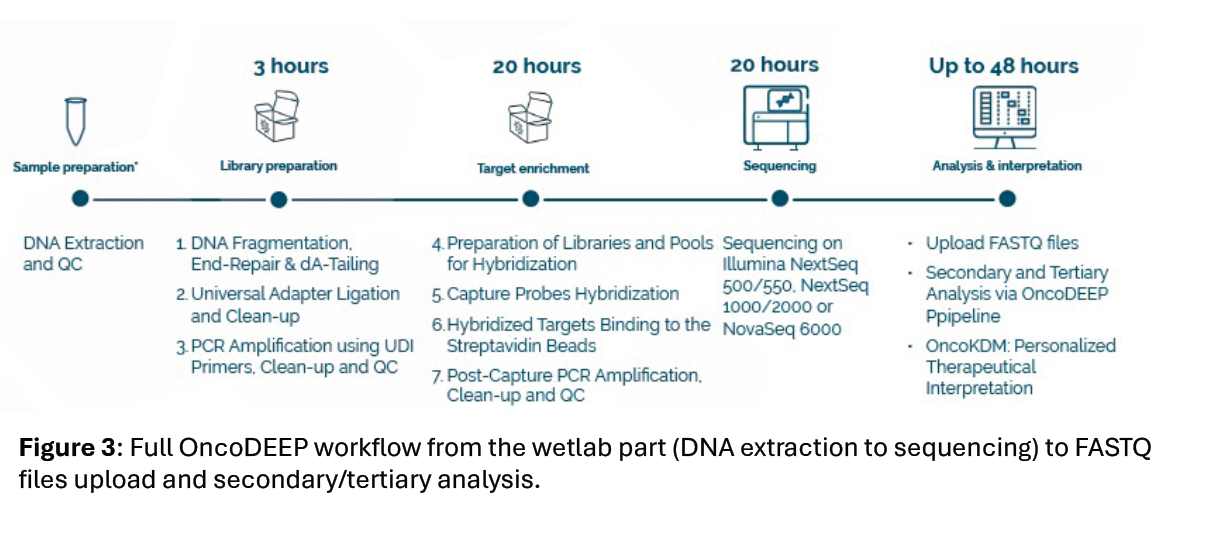

Figure 4.
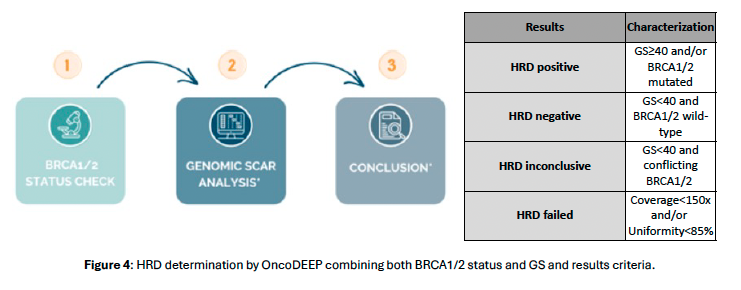

Table 1.
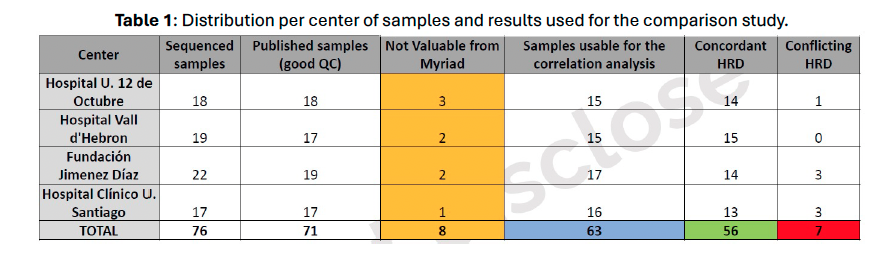

Figure 5.
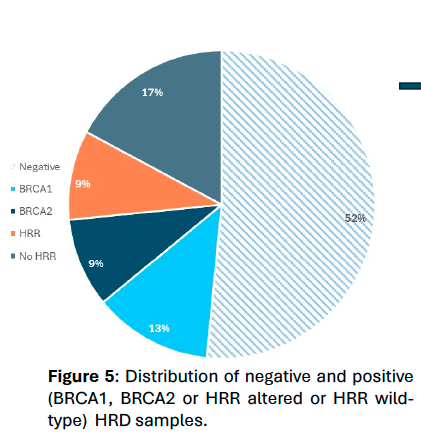

Figure 6.
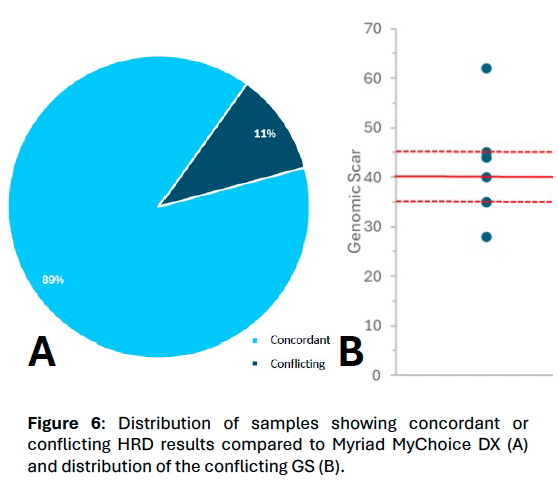

Figure 7.
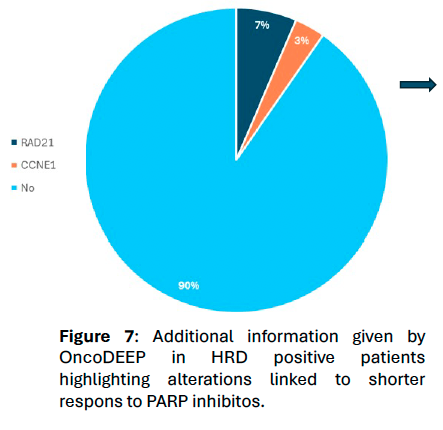

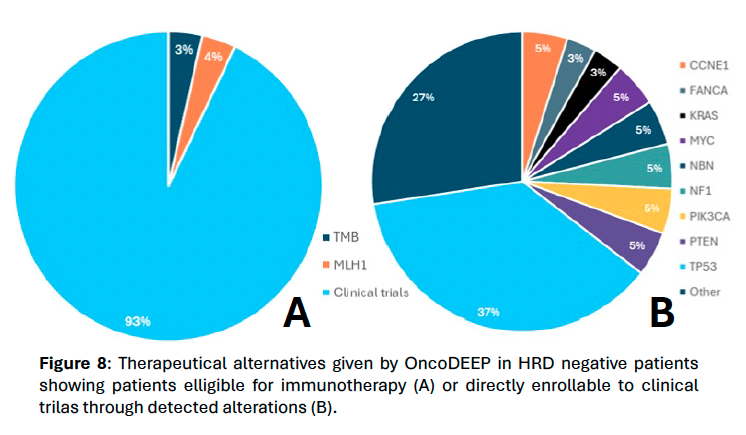
Figure 8.