What is HRD Genomic Scar?
The HRD Genomic Scar (GS) is essentially a collection of genetic changes or signatures that indicate a cell’s inability to repair DNA damage effectively using this specific DNA repair pathway.
HRD GS analysis is particularly relevant in the context of certain cancer treatments, such as PARP inhibitors. These drugs are used in the treatment of some cancers, such as ovarian and breast cancers, where HRD is common. Tumors with HRD genomic scars are more responsive to PARP inhibitors: these drugs take advantage of the fact that cancer cells have a broken or weakened “repair system” for their DNA. This makes the cancer cells vulnerable and lead to cell death.
Identifying the HRD Genomic Scar in cancer patients can help oncologists and researchers to:
- Tailor treatment from a selection of patients who are more likely to respond to PARP inhibitors and other targeted therapies, leading to more effective and personalized treatment.
- Screen patients who could be enrolled in clinical trials investigating HRD-targeted therapies, facilitating the development of new treatments.
New HRD GS classification results
To enhance the interpretation of HRD biomarkers, OncoDNA’s current approach has been slightly improved.
The HRD Genomic Scar remains unchanged. However, there has been an adjustment in the interpretation of the results to lead now to the incorporation of inconclusive result. It is given when the patient has a proficient GS associated with the detection of a genetic variant in BRCA1 or BRCA2 with a complex biological interpretation.
This new interpretation method is an evolution to align more closely with the latest scientific progress in the field and to provide even more detailed results.
This approach will be based on 3 steps:

Final results
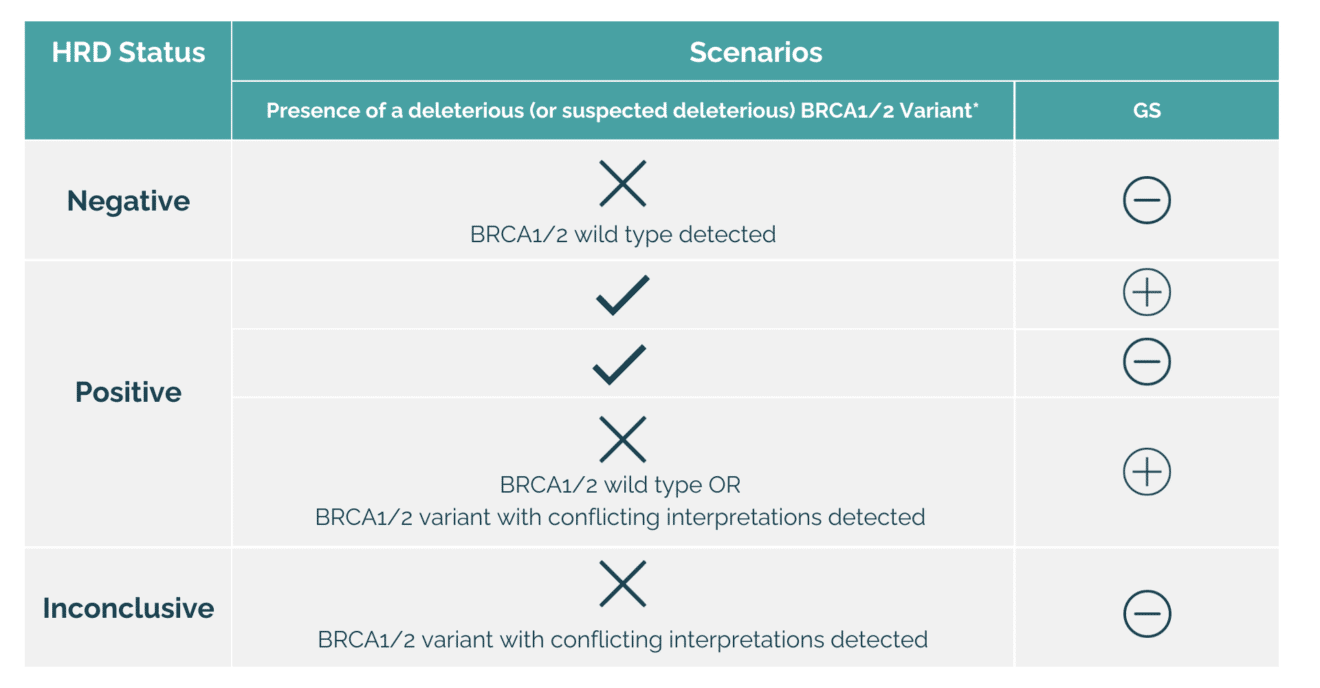
* includes variants for which published data demonstrate a loss of function of the corresponding proteins as well as the large rearrangements
HRD Genomic Scar studies play a pivotal role in the field of oncology and cancer research. Better HRD Scar classification results positively impact both patients and clinicians by enabling more personalized treatment decisions, reducing over-treatment, improving patient outcomes, and supporting informed decision-making in cancer care.
