
Case description:
Cholangiocarcinoma can be classified into subtypes depending on the primary anatomic: intrahepatic cholangiocarcinoma and extrahepatic cholangiocarcinoma (perihilar cholangiocarcinoma and distal cholangiocarcinoma).
Intrahepatic cholangiocarcinoma is the most common primary liver cancer after hepatocellular carcinoma. The early diagnosis of this cancer remains a challenge because of the silent clinical manifestation. The prognosis of both subtypes of cholangiocarcinoma is poor. Current therapeutic option remains on first complete surgical resection but the situation of the primary tumor constitutes a challenge to proceed. Indeed, small tumor can be located in difficult access localisation, multiple lesions can also be identified. The 5-year overall survival for patients with intrahepatic cholangiocarcinoma is around 10% [PMID: 33735689].
The use of adjuvant therapy is needed. The most common chemotherapy is gemcitabine alone or in combination with cytotoxic agent such as cisplatin. The better knowledge of the genomic landscape of the tumor allows the use of targeted therapies. The main two are targeting alteration in isocitrate dehydrogenase (IDH) and fibroblast growth factor receptor (FGFR). [PMID: 36260350].
We will describe the case of a 45 year old man diagnosed with an intrahepatic cholangiocarcinoma in 2022. He was treated by surgery and had several cycle of chemotherapies. The patient shows signs of resistance to the adjuvant therapy. Therefore a genomic profile of the tumor has been performed using the OncoDEEP solution.
Clinical interpretation and analysis results
IDH1 variant analysis in intrahepatic cholangiocarcinoma
The OncoDEEP panel did not identify the presence of IDH1 variant for this patient.
IDH1 alteration is found in about 20% of patients with intrahepatic cholangiocarcinoma [PMID: 37746315]. Isocitrate dehydrogenase (IDH) is a key metabolism enzyme that is responsible of the conversion of isocitrate into alpha-ketoglutarate, involved in the Krebs Cycle.
The IDH mutation usually leads to a gain of function of the protein leading to the increase of alpha-ketoglutarate conversion into D-2-hydroxyglutarate, an oncometabolite. The high concentration of the D-2- hydroxyglutarate leads to dysregulation of histone and DNA methylation (alpha-ketoglutarate enzyme dependent) promoting malignant transformation. This oncometabolites also lead to the promotion of cellular proliferation by stabilisation of the hypoxia-inductible factor 1 alpha (HIF-1α) [PMID: 33182517 ; PMID: 37746315].
Ivosidenib (also called AG-120) is an inhibitor of IDH1, FDA approved drug for patients with IDH1 mutation and previously treated locally advanced or metastatic cholangiocarcinoma. The Ivosidenib allows to decrease the level of D-2-hydroxyglutarate to restore the level of alpha-ketoglutarate and the activity of alpha-ketoglutarate enzymes dependent, involved in the regulation of histone and DNA methylation [PMID: 33683991]. For this patient, Ivosidenib is therefore not recommended since there is no mutation in IDH1.
Detection of FGFR2-BICC1 fusion and its implications
Nevertheless, we detected a fusion FGFR2-BICC1 with our OncoDEEP RNA panel. Indeed, our oncoDEEP solution includes a DNA panel allowing us to identify genomic alterations but also a RNA panel targeting splicing event and fusion in 22 genes involved tumor development. The detection of gene fusion is performed by specific primers pairs designed for the identification of rearrangements.
A fusion FGFR2 – BICC1 has been identified in the RNA for this patient.
Fibroblast growth factor receptor (FGFR) family belongs to the family of the tyrosine kinase receptors. There are four family member: FGFR1, FGFR2, FGFR3 and FGFR4. Those receptors are involved in several cellular processes such as proliferation, survival, migration, angiogenesis. FGFR signalling pathways is triggered by the binding of the ligand that allows the recruitment of intermediate proteins leading to the activation of the downstream pathway. FGFR fusion lead to a constitutive activity of the receptor independent of the ligand. FGFR2 fusion is characteristic of the intrahepatic cholangiocarcinoma. About 14% of cholangiocarcinoma shows a FGFR2 fusion [PMID: 23900974 ; PMID: 36497187 ; PMID: 29848569].
The FGFR receptor is composed by 3 domains:
- the extracellular domain that allows the ligation of the ligand,
- the transmembrane domain and
- the intracellular domain which contains the tyrosine kinase activity.variant
In case of FGFR2 fusion, the extracellular domain and the kinase domain is conserved. The fusion partner, in this case BICC1, allows dimerization signal and lead to the activation of the downstream pathway. FGFR2 translocation can be targeted by tyrosine kinase inhibitor [PMID: 32983265]. Pemigatinib, an oral selective inhibitor, is approved for the treatment of cholangiocarcinoma with FGFR2 fusion. [ PMID: 36497187].
In conclusion, the OncoDEEP kit analysis reveals the absence of mutation in IDH1 but highlight the detection of an FGFR2 fusion. The treatment of intrahepatic cholangiocarcinoma remains a challenge and is still associated with poor prognostic for the patient. The use of targeted therapy such as FGFR2 inhibitors paved the way to a better chance of survival for the patient.
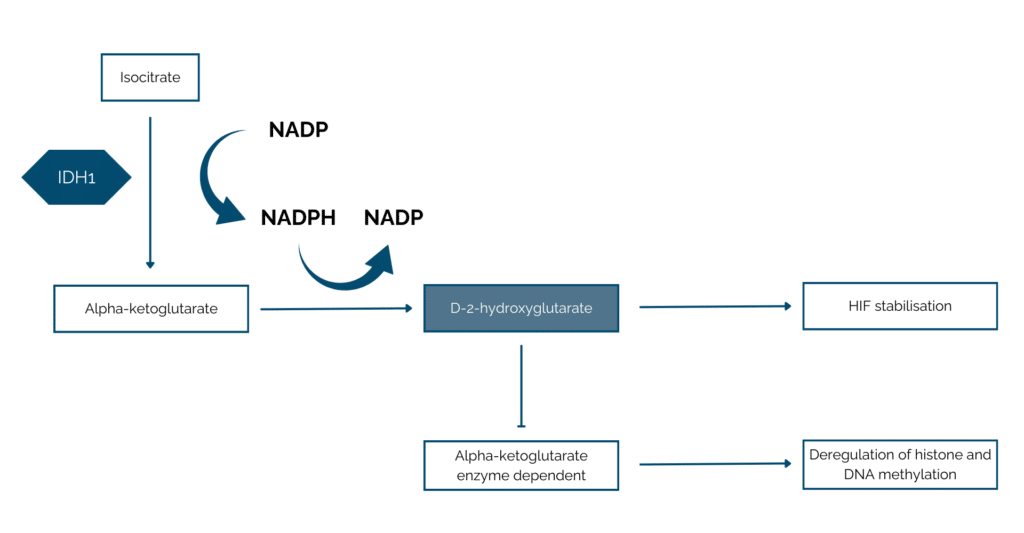
Fig : Isocitrate is converted into alpha ketoglutarate by the Isocitrate dehydrogenase in normal condition. In case of IDH1 mutated, the alpha-ketoglutarate is converted into an oncometabolite, D-2-hydroxyglutarate. The accumulation of this oncometabolite leads to dysregulation of alpha-ketoglutarate enzyme dependent which leads to dysregulation of histone and DNA methylation. In addition, the HIF factor is also stabilised by the high concentration of D-2-hydroxyglutarate.
